For a fast and safe stroke assessment
BrainWave is a novel device for brain stroke detection and classification
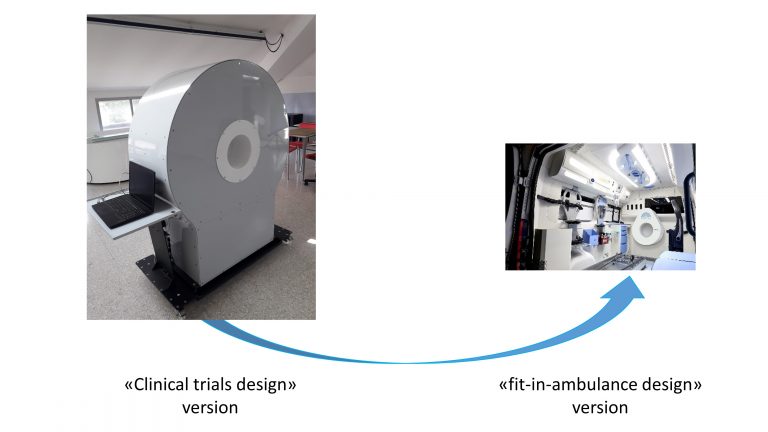

BrainWave is a novel device for brain stroke detection and classification. BrainWave uses a wave-guided device configuration, containing two antennas that operate in the microwave band (1-5 GHz) at a very low power (1mW). BrainWave allows to illuminate the head using an electromagnetic field and to measure the correspondent scattered fields. The measured scattered field is then processed through a dedicated algorithm, obtaining one or more images of the brain Microwave imaging exploits the contrast in the dielectric properties at microwave frequencies of normal tissues and tissues with lesions (such as haemorrhagic/ischaemic stroke). BrainWave software uses a novel, fast, and accurate imaging algorithm (based on the Huygens Principle) which highlights tissues dielectric inhomogeneities.
clinical validation is ongoing with the aim of assessing performance in discriminating between haemorrhagic and ischaemic stroke
our solution is non-ionizing, meaning there is no radiation risk. Examination is performed using safe low-power radio-frequency signal in the microwave band.
Examination and results are performed and obtained in few minutes
BrainWave can be fitted into ambulances, allowing to perform a pre-hospital stoke detection and classification

Do not hesitate to contact us. We will be happy to inform you about our innovative medical devices

Santa Maria della Spina Str., 25
06081 Rivotorto di Assisi (PG) Italy
VAT: 03434740548
Tel: +39 3490564302
gianluigi@ubt-tech.com (Scientific)
g.raspa@ubt-tech.com (Hardware)
s.tiberi@ubt-tech.com (Business)
amministrazione@ubt-tech.com (Administration)

3rd Floor 207 Regent Street, London, W1B 3HH (Registered)
103 Borough Rd, London, SE1 0AA (Operational)
Company number 13096679
According to the Italian Ministry of Health guidelines of 28/03/2013, the information contained in this website is exclusively intended for professional operators.